An ion has an unequal number of protons and electrons. The atomic mass is in the upper right corner.

Answered 10 Name The Element Which Has The Bartleby
E86electrons125neutrons82protons charged atom f0 neutrons.
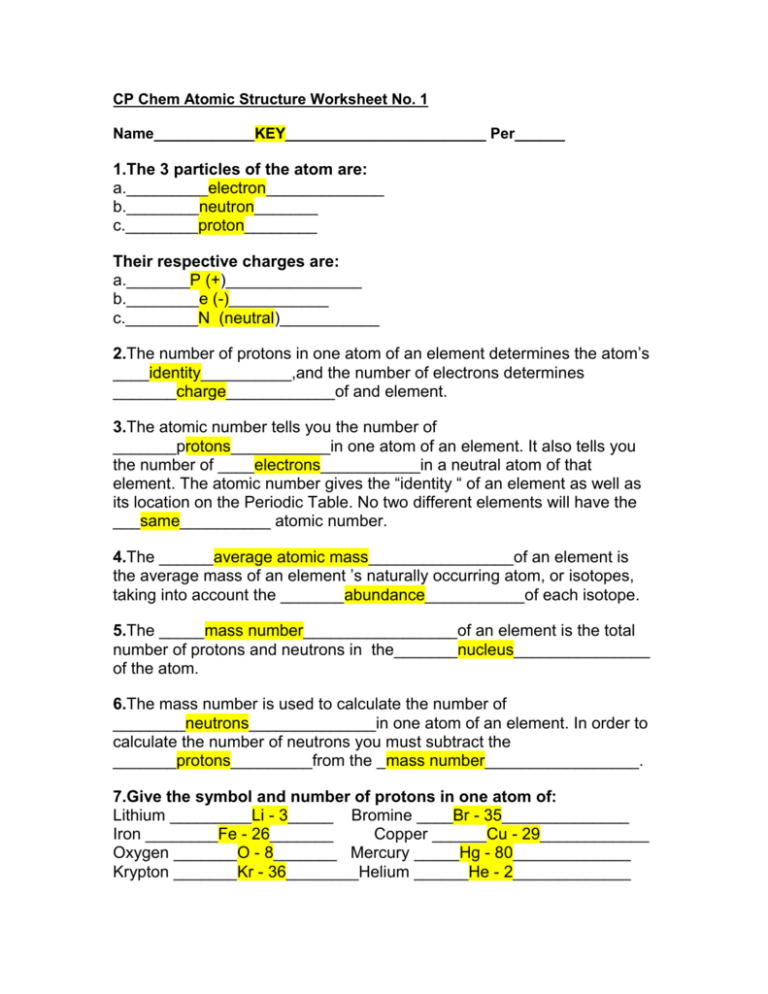
. Include charges and mass numbers where possible 26 electrons 29 neutrons 26 protons 53 protons 74 neutrons 2 electrons neutral atom 20 protons 86 electrons 125 neutrons 82 protons charged atom O neutrons. If the charge is positive there are more protons than. A26 electrons29 neutrons26 protons.
Name the elementwhich has the following numbers of particles. Number of protons atomic. Number of efectrons in a neutral atom d.
All iron atoms and isotopes have the same number of protons but different number of neutrons. A neutral atom has the same number of protons and electrons charges cancel each other out. An atom wih 25 protons is Manganese or an isotope of manganese.
Chemistry questions and answers. Name the elementwhich has the following numbers of particles. View the full answer.
Number of electrons in a neutral atom d. 26 electrons 29 neutrons 26 protons 53 protons 74 neutrons 2 electrons neutral atoms 20 protons 86 electrons 125 neutrons 82 protons O neutrons 11. 30 What is the element with 8 protons.
If an atom has 17 electrons and its mass number is 35 calculate the following. If you know ONLY the following information can you ALWAYS determine what the element is. Number of neutrons c.
Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. 112 rows Manganese has 25 protons 30 neutrons and 25 electrons. D 26 protons 31 neutrons and 29 electrons 2 When barium metal reacts with from CHEM 101 at Gateway Community College.
A neutral iron atom contains 26 protons and 30 neutrons plus 26 electrons in four different shells around the nucleus. 28 Does calcium have 2 valence electrons. There are 26 protons and 30.
Ff you know ONLY the following information can you ALWAYS determine what the element is. Question 4 1 pts An ion has 26 protons 29 neutrons and 23 electrons. If you know ONLY the following information can you ALWAYS determine what the element is.
The symbol for the ion is What is. As with other transition metals a variable number of electrons from irons two outermost shells are available to combine with other elements. The symbol for the ion is.
52Cu3 5573- 55Fe3 52Cu3- 55Fe3- D Question 5 1 pts An atom that has an atomic number of 38 and a mass number of 88 is an isotope of an atom that has an atomic number of 39 and a mass number of. C2electrons neutral atom d20 protons. Iron has 26 protons 30.
30 neutrons and 30 electrons a hydrogen monoiodide b hydrogen iodide c hydroiodic acid b 26 protons 31 neutrons and 23 electrons d monohydrogen monoiodide c 29 protons. 86 electrons 125 neutrons 82 protons f. 26 electrons 29 neutrons 26 protons b.
2 electrons neutral atoms d. 35 How to find the Number of Protons Electrons Neutrons for Calcium. The atom with 26 protons 28 neutrons 26 electrons is another iron atom.
Number of electrons 12. An ion has 26 protons 29 neutrons and 24 electrons. A26 electrons29 neutrons26 protons b53 protons74neutrons c2electronsneutral atom d20 protons e86electrons125neutrons82protonscharged atom f0 neutrons.
Number of protons b. An ion has 26 protons 29 neutrons and 24 electrons. Atoms are made of protons neutrons and electrons.
Question 27 1 point Name the element which has 26 electrons 29 neutrons 26 protons Fe V Cs Cu Question 28 1 point Name the atom or ion with the following number of particles 78 electrons 125 neutrons 82 protons Pb4 Pb Opt Po2 Question 37 1 point The correct number of significant figures in. 53 protons 74 neutrons c. 26 How many neutrons are in CR 53.
29 What is the atom of calcium. 27 How many electrons does calcium 43 have. 26 electrons 29 neutrons 26 protons 53 protons 74 neutrons 2 electrons neutral atoms 20 protons 86 electrons 125 neutrons 82 protons O neutrons 11.
The number of protons gives us an idea of what atom it is. An iron atom has 26 protons. The symbol for the ion is What is the molar mass of following biomolecule.
Number of neutrons c. Commonly iron uses two oxidation state 2 or three oxidation state 3 of its available electrons to form. Number of protons b.
The element that has 26 electrons 29 neuetrons and 26 protons is Iron Fe The number of protons electronsatomic number. Mass number is simply the total number of massive particles in the atom protons and neutrons.

Cp Chem Atomic Structure Worksheet No Ericksoncpchem2010 11

Review Atoms Name Mr John Date
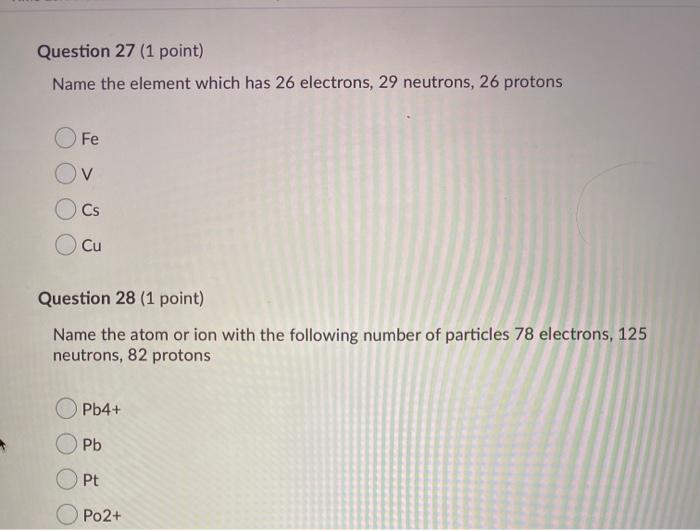
Solved Question 27 1 Point Name The Element Which Has 26 Chegg Com

Solved 1 The Fe3 Ion Has A 26 Electrons 26 Protons And Chegg Com
0 Comments